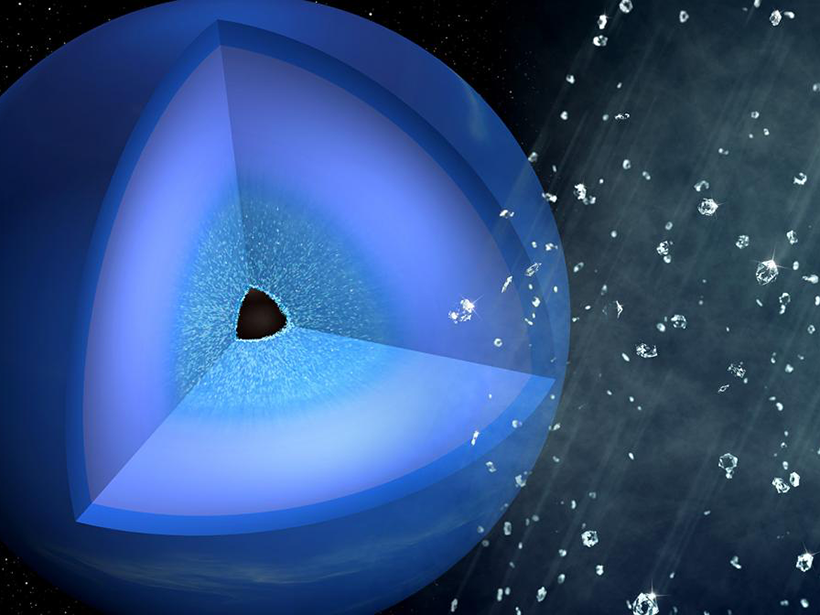
Researchers subjected hydrocarbon samples in a laboratory to Neptune-like pressures. The samples, reminiscent of molecules found in the ice giant’s atmosphere, compressed into nanodiamonds.
diamonds
Posted inNews
Mineral Flaws Clarify How Diamonds Form
A study of nanoscale, iron- and sulfur-rich impurities in diamonds provides new clues to the chemical processes that produce the superhard crystals and at what depths they occur.