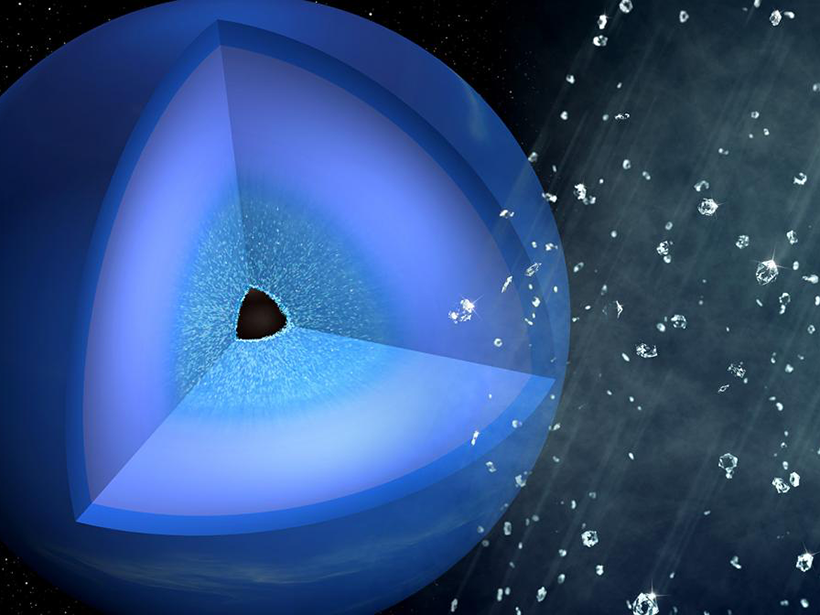
A very hard rain likely falls inside Uranus and Neptune.
In recent high-energy laser experiments, researchers have replicated the pressures and temperatures found deep in the atmospheres of such planets, known as ice giants. Those extreme conditions in the laboratory compressed hydrocarbon plastics, chemically similar to the methane found in ice giants, into tiny diamonds, giving an experimental boost to a long-standing theory about the characteristics of ice giant planets.
“It was a very surprising experiment,” said Dominik Kraus, a researcher at Helmholtz-Zentrum Dresden-Rossendorf in Dresden, Germany. His team had expected very small signs of molecules splitting apart after being subjected to high pressures, “maybe some little hints of diamonds,” he explained.
But instead, they found a very strong signal that under intense pressures, hydrocarbons in Neptune would transform into diamonds. Kraus is lead author of a 21 August Nature Astronomy paper describing the results.
Pressure Shock
In the recent tests, the experimenters first had to find a substance that was chemically similar to methane (CH4), a molecule believed to be in about 1.5% abundance on Neptune and the planet’s most common component after hydrogen and helium. They hit upon polystyrene (C8H8) plastic—not only is it a common material, but it is also easier to use because it’s a solid at room temperature, whereas methane is gas that would need to be contained.
The researchers then fired two short, but intense, pulses from a high-energy X-ray laser at the polystyrene sample. The two laser bursts hitting the sample at nearly the same time exerted a pressure shock almost 1.5 million times greater than Earth’s surface atmospheric pressure yet kept the temperature below the melting point of diamonds.
The researchers witnessed carbon separating from hydrogen and compressing into nanometer-sized diamonds.
The fleeting shock simulated conditions found around 10,000 kilometers below the surfaces of Uranus and Neptune. Then, using X-ray diffraction measurements to continually monitor the chemical nature of the sample after the laser strikes, the researchers witnessed carbon separating from hydrogen and compressing into nanometer-sized diamonds.
Carrying the experiment’s analogy back to Neptune, the results indicated that hydrocarbons known to be within Neptune likely condense into a solid as you go deeper into the planet’s interior. The experiments took place at the Stanford Linear Accelerator Center (SLAC) National Accelerator Laboratory in Menlo Park, Calif.
Why Simulate Neptune’s Atmosphere?
Knowing how hydrocarbons might behave deep within an ice giant’s atmosphere will affect our understanding of how atmospheres transport heat and evolve over time, explained Kraus. What’s more, the implications of this research extend beyond our solar system to exoplanets, as a large fraction of the known exoplanets are similar in size or mass to our ice giants.
The ability to model an ice giant atmosphere’s density from the top down to the core is a critical part of characterizing that planet. For example, an atmosphere made mostly of hydrogen is much puffier than one with diamonds, Kraus noted.
A diamond-studded atmosphere also likely behaves very differently than one without diamonds. For example, atmospheric convection might have to overcome more hurdles, which may lead to sharp changes in chemical composition between different atmospheric layers, the researchers said. This could also inhibit heat flow.
“These experiments can be used to improve our understanding of the behavior of common materials in the universe at high pressures and temperatures, which has a direct connection to modeling planetary interiors,” said Ravit Helled, a computational science and theoretical astrophysics professor at the University of Zurich in Switzerland, who was not involved in the study.
One-Two Punch Keeps Old Theory in the Ring
The one-two X-ray punch was the key to the experiment’s success.
Planetary scientist Marvin Ross first proposed the idea that Uranus and Neptune could have diamond precipitation in 1981. Other research groups have tried many times since then to observe this chemical reaction in the lab but have seen only hints of hydrogen-carbon separation and diamond formation. Moreover, these changes took place at pressures and temperatures that don’t match theory very well.
In contrast, the shock method used by Kraus and his team produced strong signals from the separation and diamond formation at the temperatures and pressures suggested by theory. The one-two X-ray punch was the key to the experiment’s success, according to coauthor Siegfried Glenzer, professor of photon science at Stanford University and director of SLAC’s High Energy Density Sciences Division.
“We saw carbon clusters forming under the presence of hydrogen, and then we saw those carbon clusters forming diamonds under high pressure,” Glenzer described.
Kraus added that “nearly every carbon atom inside the plastic turned, within this 1 nanosecond or less, into a diamond crystal structure.” He said that if the nanometer-scale diamonds could grow for longer spans of time, like they might in ice giant atmospheres, the nanodiamonds “would for sure grow to much larger size.”
To see diamond formation, the sample needed to be highly compressed but not heated beyond the melting point of diamonds, a tricky combination for most laser experiments, Glenzer explained. The lasers compressed the sample for only a few nanoseconds, too short a time to substantially increase the temperature. The team performed its experiment with the Matter in Extreme Conditions (MEC) instrument on SLAC’s Linac Coherent Light Source (LCLS).
SLAC’s full range of experimental techniques allows scientists “to be able to assess these questions of reactivity and kinetics,” said Laura Robin Benedetti, an experimental physicist at Lawrence Livermore National Laboratory in Livermore, Calif., who did not participate in the research. “It’s very exciting to have new work in this field.”
The Hydrogen Solution
“Nearly every carbon atom inside the plastic turned, within this 1 nanosecond or less, into a diamond crystal structure.”
Glenzer explained that the shock method’s short timescale is important for keeping hydrogen from escaping during the diamond compression. Past experiments that observed the reactions over the course of a few seconds might have suffered from hydrogen loss, he speculated.
In the shock experiments, “hydrogen is still present, and that’s important because that’s what happens [in] Neptune,” Glenzer emphasized. “You have carbon under high pressure, and hydrogen is still around. And then we see the formation of diamonds.” In this way, the lab simulation “is a much better approximation for what we believe is happening in Neptune.”
“Hydrogen is still present, and that’s important because that’s what happens [in] Neptune.”
The research group has begun conducting similar experiments with plastics of different composition to test the range of reactions that could occur, according to Kraus. He and his colleagues are particularly interested in reactions that include oxygen and helium, two elements in high abundance in not just ice giants but also Jupiter-like planets as well.
“To refine our models of the interiors of the ice giant planets and also to understand their formation processes, we will need every bit of data we can get our hands on!” Benedetti told Eos in an email.
The team also hopes to retrieve the newly formed diamonds from the MEC chamber to analyze their structure and strength, Glenzer said. Through that, there may be a practical application to this science: Harvesting the diamond nanocrystals formed in the experiments is the researchers’ first step in assessing potential applications for the diamonds in material science or industry.
—Kimberly M. S. Cartier (@AstroKimCartier), News Writing and Production Intern
Citation:
Cartier, K. M. S. (2017), Diamonds really do rain on Neptune, experiments conclude, Eos, 98, https://doi.org/10.1029/2017EO082223. Published on 15 September 2017.
Text © 2017. The authors. CC BY-NC-ND 3.0
Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.