Through roving and drilling, Mars Curiosity Rover may be breaking up the ground’s salty, hardened soils that seal methane, possibly causing a temporal, local methane spike.
Yasuhito Sekine
Associate Editor, JGR: Planets
Posted inEditors' Highlights
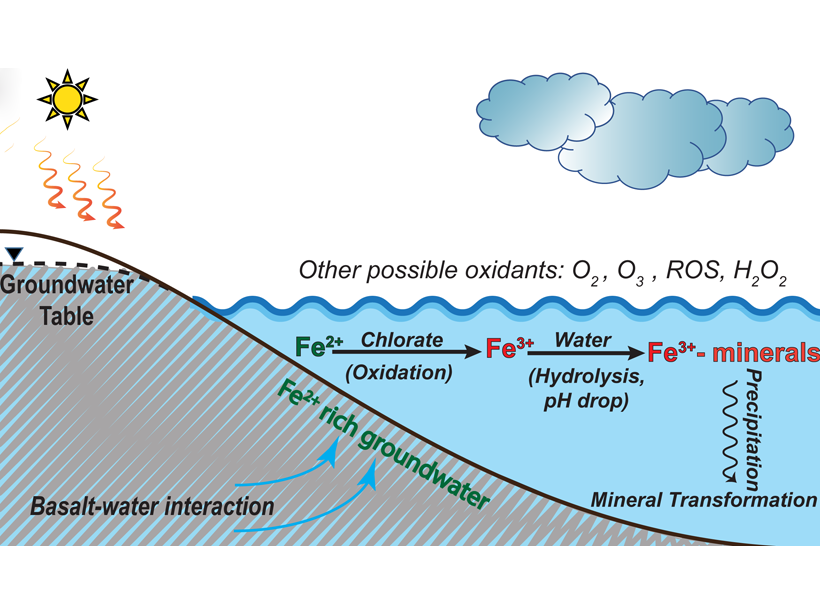
Why Is the Red Planet Red? Chlorate May Oxidize Mars’ Surface
Laboratory experiments and geochemical model suggest that chlorate is very effective to oxidize reducing iron to reddish iron oxides on Mars when liquid water was present on the surface.