Source: Journal of Geophysical Research: Planets
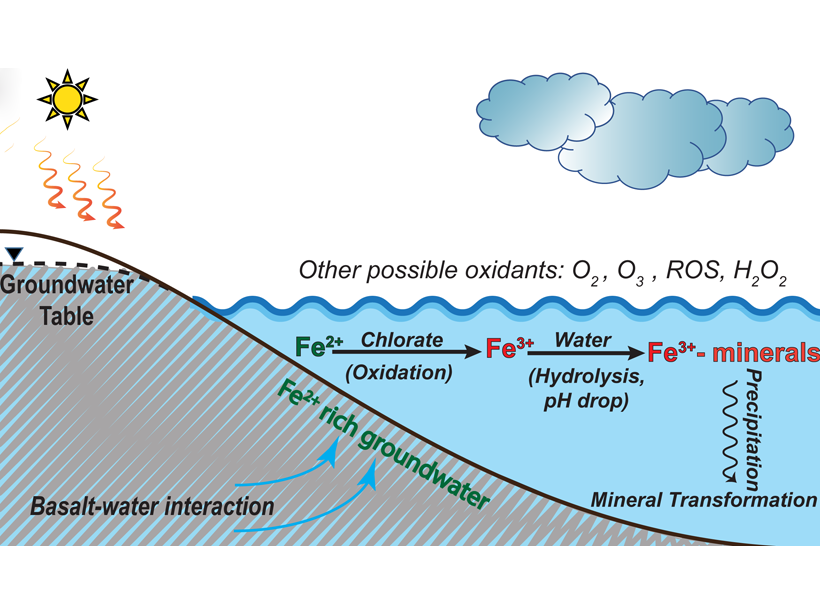
Why is the red planet red? It is well known that it is iron oxides that color Mars’ surface a reddish tint; however, the process(es) responsible for the oxidation of iron on Mars remain unknown.
Based on laboratory experiments, Mitra and Catalano [2019] provide a new insight into oxidation of iron on Mars. They focus on oxychlorine species, salts of chlorine and oxygen, that are globally distributed on Mars. Having compared with other oxidants, such as molecular oxygen, they suggest that chlorate, one of oxychlorine species, was effective to oxidize reducing iron in various aqueous environments on Mars.
Since life usually requires an oxidant to obtain energy, the presence of effective oxidant, such as chlorate, on early Mars also has strong implications for its habitability.
Citation: Mitra, K., & Catalano, J. G. [2019]. Chlorate as a potential oxidant on Mars: Rates and products of dissolved Fe(II) oxidation. Journal of Geophysical Research: Planets, 124. https://doi.org/10.1029/2019JE006133
—Yasuhito Sekine, Associate Editor, JGR: Planets
Text © 2019. The authors. CC BY-NC-ND 3.0
Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.