Imagine you could go back in time to see what life was like in the sulfur-rich, oxygen-poor seas of Earth’s Proterozoic eon (~2.5–0.6 billion years ago). No one knows exactly what ecosystems of the distant past looked like, but there’s a good chance that you’d see thin veils of microbial life clinging to shallow sunlit sediments or thriving around deep-sea hydrothermal vents.
As it happens, geology, water, and time have converged to paint colorful microbial mats across underwater landscapes in North America’s Great Lakes (and elsewhere) that are thought to resemble the planet’s early biosphere. Similar mat communities may also exist and mark the beginnings of life on other habitable planets or moons.
Around the world, isolated aquatic environments with low oxygen and high sulfur levels provide refugia that enable such modern-day mats to thrive. These communities of extremophiles, such as those in sinkholes at the bottom of Lake Huron, contribute greatly to our understanding of the origins of life on Earth. They also add to Earth’s biodiversity, provide relatively easy to access analogues of deep marine vents, and can serve as test beds in our search for alien life.
Recent research has continued to reveal fascinating and vital aspects of microbial mats that survive under extreme environmental conditions. For example, findings have indicated that Lake Huron’s sinkholes are carbon sinks, that mat microbes may optimize oxygen production through daily migrations of less than a millimeter, and that more submerged sinkholes are present across the Great Lakes basin than previously thought.
These unique ecosystems have many more secrets to reveal and insights to offer about past, present, and maybe future life on Earth, as well as about the conditions under which life might survive on habitable extraterrestrial worlds. However, they are also vulnerable to damage from natural and anthropogenic pressures, suggesting an important need not only to further study them but also to conserve them.
Mats of the Great Lakes
Today, microbial mats find refugia in globally distributed extreme environments such as cold seeps, thermal springs, permanently ice covered lakes in the dry valleys of Antarctica, submarine blue holes, and deep-sea vents.
In 2001, maritime archaeologists exploring Thunder Bay National Marine Sanctuary in Lake Huron made the first of many discoveries of submerged sinkholes in the lake.
In 2001, maritime archaeologists exploring Thunder Bay National Marine Sanctuary in Lake Huron made a serendipitous finding of what appeared to be limestone karst features. This was the first of many discoveries of submerged sinkholes in the lake, in everywhere from sunlit shallows to its deep, dark bottom waters. Further studies have revealed that these groundwater-fed sinkholes contain relatively dense, salty, acidic, and cold water compared with the typical fresh lake water above [Biddanda et al., 2012]. This venting groundwater spreads over the lake bottom, fueling growth of vibrantly colored microbial mats.
All four lower Great Lakes (from west to east: Michigan, Huron, Erie, and Ontario) have foundations of roughly 400-million-year-old Paleozoic limestone karst. Groundwater in their aquifers washes through marine evaporites, gaining sulfates and losing oxygen during its transit underground to springs and sinkholes. Over time, this water has eroded the limestone underneath the Great Lakes to form submerged sinkholes.

These Great Lakes sinkholes are hard to find because they are relatively small—ranging from the size of a coffee table to a soccer field—and far apart (meters to kilometers) and because there are no signatures of their presence on the lakes’ surfaces. Yet they may be abundant in all four lower Great Lakes given their similar bedrock. Indeed, multibeam acoustic surveys by our team in the past 6 years have revealed more than 2 dozen previously unmapped sinkholes in offshore Lake Huron at depths greater than 120 meters. We also recently explored the Great Sulphur Spring, a sinkhole about half the size of a soccer field in the Lake Erie basin containing cyanobacterial mats similar to those in Lake Huron sinkholes.
Meanwhile, colleagues at Wisconsin Shipwreck Coast National Marine Sanctuary have recently reported numerous lake bottom anomalies in western Lake Michigan that are hallmarks of sinkholes. And sport divers have made many reports of mat sightings in Lake Ontario and Lake Erie, although these reports have not yet been investigated scientifically.
Clues to the Biosphere’s Beginnings
Microbial life in many of these sinkholes is characterized by a textbook redox tower, a series of reactions extending from organisms on the mat surface down to microbes in the sediments below.
Microbial life in many of these sinkholes is characterized by a textbook redox tower, a series of oxidation-reduction reactions extending from organisms on the mat surface that make their own food in oxygen-free as well as oxygen-rich water (anoxygenic and oxygenic autotrophs, respectively) down to methane-generating microbes in the oxygen-free sediments below (methanogenic chemoautotrophs) [Biddanda et al., 2012]. Photosynthetic and chemosynthetic mats colonize shallow, sunlit sinkholes. In deeper water, where sunlight cannot penetrate, sinkholes are inhabited by chemosynthetic mats similar to those found around submarine seeps in the ocean where sulfur-rich water is venting.
Knowledge of the rate and timing of photosynthesis through Earth’s deep past is critical to understanding how the planet’s atmosphere became oxygenated. Infant Earth’s atmosphere contained essentially zero oxygen, but geologic evidence suggests that beginning about 2.5 billion years ago, oxygen slowly accumulated to concentrations that allow us to live and breathe today [Klatt et al., 2021].
Many theories have been proposed to explain the slow pace of oxygenation from about 1.8 billion to 0.8 billion years ago—the so-called Boring Billion—when seemingly little changed in the planet’s climate, chemistry, and biology. One theory, for example, posits that the younger Earth’s short day length (resulting from its faster rotation) limited the amount of photosynthesis that could occur over a continuous period of sunlight. As the tidal pull of the Moon slowed the planet’s spinning and day length increased, eventually to the 24 hours we experience now, the daily duration of photosynthesis also likely increased, resulting in net oxygenation of the water as well as the air.
Today, slow rates of oxygen production reminiscent of the Proterozoic are exemplified in shallow sinkholes where photosynthesis is closely balanced by respiration. Only late in the day does oxygen production outpace its consumption in these environments. Models suggest that such meager, but measurable, net oxygen production would have oxygenated the atmosphere over eons as Earth’s day length increased [Klatt et al., 2021].
Microbial Choreography
The everyday lifestyle of today’s sinkhole microbes offers clues to how the early biosphere might have worked to bury organic carbon and emit oxygen.
The everyday lifestyle of today’s sinkhole microbes offers clues to how the early biosphere might have worked to bury organic carbon and emit oxygen. In what might be a modern incarnation of the first synchronized movement ever choreographed, photosynthetic and chemosynthetic microbes alternately migrate hundreds of micrometers daily at speeds of 50–200 micrometers per minute toward the mat surfaces. There, they take turns harvesting sunlight and hydrogen sulfide during the day and oxygen at night, turning the mats distinctly purple during the day and white at night [Biddanda et al., 2023].
Were similar 1-millimeter journeys the largest daily mass movements of life during the long Proterozoic? Were they responsible for optimizing mat building, photosynthesis, chemosynthesis, carbon burial, and oxygen release in the early biosphere? Synchronized diel (daily) vertical migration provides a potential mechanism for maximizing these processes.
Evidence so far suggests that sinkholes are, indeed, sinks for carbon, with mat microbes’ daily migrations amplifying the biological pump’s removal of surface carbon into sediments. Nold et al. [2013] revealed that the huge repository of organic matter–rich sediments underlying sinkhole mats were primarily composed of planktonic carbon. Each day, vertically migrating phototactic (i.e., moving toward light) mat filaments climbed over settling organic carbon debris from above (such as dead plankton) and subducted it into the anaerobic layer below, where it was preserved.

In another study, pebbles and shells placed atop mats were covered by mat filaments climbing over them within hours, and they were completely buried out of sight beneath sediments within days [Biddanda et al., 2015]. Biomarker studies in sulfur spring-fed Lake Cadagno in Switzerland also have shown evidence for high rates of organic carbon accumulation under microbial mats [Hebting et al., 2006].
Contributions to Modern-Day Biodiversity
Microbial mats in Lake Huron’s sinkholes comprise a diverse assemblage of bacteria, archaea, and viruses. A small number of protists and even low-oxygen-tolerant invertebrates such as tardigrades and nematodes live at the periphery of sinkholes where groundwater mixes with lake water. Primary producers in the mats include motile filamentous cyanobacteria that carry out both anoxygenic and oxygenic photosynthesis, as well as microbes that chemosynthesize by oxidizing sulfur. Sediment layers below the mats provide niches for sulfate-reducing bacteria, methanogens, and a variety of other organisms.
Consortium-lifestyle microbial mats offer ideal contexts for studying ecological, biogeochemical, and evolutionary interactions. For example, researchers have probed the long and intimate relationship between mat cyanobacteria and their viruses. Voorhies et al. [2015] directly linked an abundant virus to its cyanobacterial host in a modern-day mat ecosystem. Such interactions between the organisms may reenact some of the very first microscopic biologial warfare, which might have had profound outcomes for life over its long geobiologic history.
Mat organisms’ ability to thrive in extreme environments makes microbial mats practical analogues and test sites in our search for life in extraterrestrial hydrospheres.
The mats also enable robust testing of the effects of biodiversity on ecosystem stability and of concepts like evenness–functional redundancy relationships. Indeed, omics studies (e.g., genomic, metabolomic, and, especially, proteomic) of mat community compositions suggest that these communities are functionally diverse and versatile [Voorhies et al., 2012; Grim et al., 2021; McGovern et al., 2023].
Mat organisms’ ability to thrive in extreme environments, including below winter ice cover in Great Lakes sinkholes, makes these mats practical analogues and test sites in our search for life in extraterrestrial hydrospheres [Biddanda et al., 2021]. In addition, they could harbor secondary metabolites of considerable pharmacological potential.
Another fascinating feature of these mats is distinctive conical protrusions filled with sedimentary gases such as methane that form near the peak growing season in summer. In the field, we have observed that these protrusions frequently tear off and float to the surface during late summer, where water and wind currents may disperse them. And in very recent laboratory studies, we have observed nonmotile filaments (of both cyanobacteria and sulfur-oxidizing microbes) in dehydrated mats become motile again within minutes of rehydration by groundwater. This observation, if confirmed, hints that transport by water and air could be a mechanism by which mat microbes have colonized refugia from the tropics to the poles.
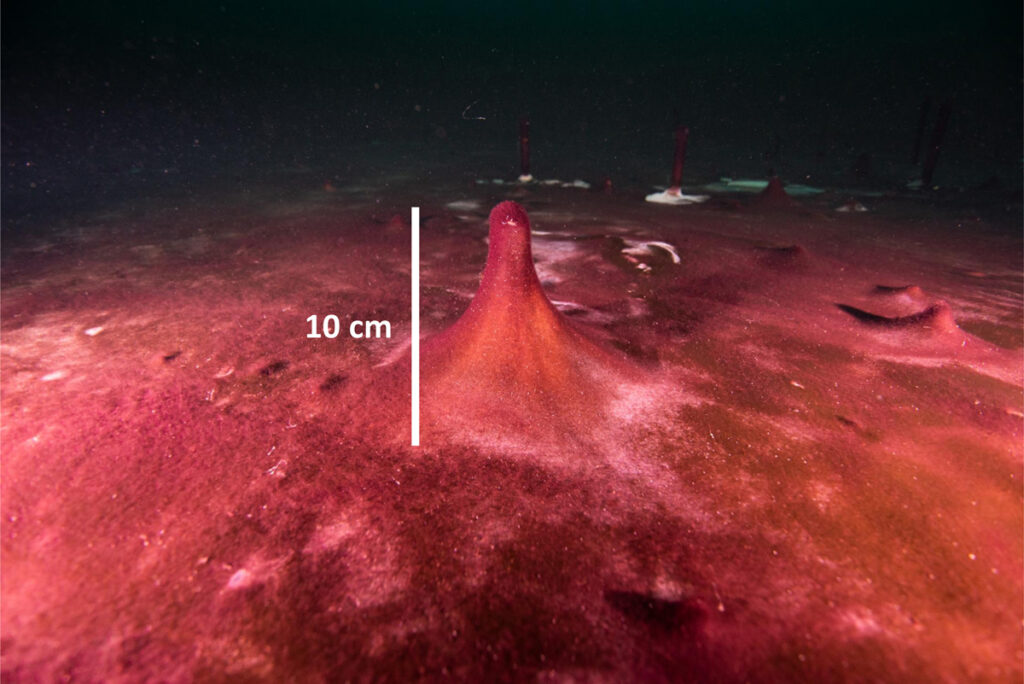
The collective contribution of groundwater-fed sinkholes to the biodiversity and physiologies of the Great Lakes, as well as to the lakes’ water quality and even water levels, makes them important features. But the roles and significance of extant microbial mats thriving under extreme environmental conditions around the world remain largely unnoticed—and their vulnerability to environmental perturbations remains unquantified.
The potential for these environments to harbor clues to our evolutionary past and the fact that many basic scientific questions about their present-day structure and function remain unanswered support the need for their conservation.
The Vulnerability and Value of Mats
Mat ecosystems require protection because of their relative isolation, rarity, biological uniqueness and diversity, and likely outsized role in shaping the biosphere’s past and present—and possibly its future because it’s not inconceivable that Earth’s biosphere could someday be dominated by mats again if conditions for nonmicrobial life become untenable. Today these ecosystems are vulnerable to climate change and anthropogenic pressures from hydroengineering of watersheds and paving of on-land areas that recharge offshore sinkholes, for example. Holistic and sustainable conservation of submerged, mat-hosting sinkholes should involve protection of both terrestrial and aquatic domains that influence them.
Several anthropogenic perturbations may affect these systems in unknown ways, whereas others pose more obvious threats. We already know, for example, about the negative impacts of herbicides on photosynthetic communities of lakes and coastal ocean habitats such as corals, but we don’t know how sensitive isolated sinkhole microbial communities may be to such pollution. We also don’t know the age of groundwater entering Great Lakes sinkholes; the younger the water is, the greater the concern is that effects of terrestrial perturbations on mat ecosystems may be more immediate.
In addition to more direct hazards, climate change may cause indirect threats to microbial mat ecosystems because of its effects on everything from water temperatures and ice cover to wind and rainfall.
Other direct threats to these ecosystems emerge from potential industrial activities such as exploration for lake bed oil, gas, or minerals as well as offshore wind turbine siting, among others. Although sinkholes like those within Thunder Bay National Marine Sanctuary are relatively protected from such direct threats, sinkholes elsewhere across the lower Great Lakes are not afforded the same assurance.
In addition to more direct hazards, climate change may cause indirect threats because of its effects on everything from water temperatures and ice cover to wind and rainfall. Thus, it raises many conservation-related questions: How might climate change affect the survival of isolated and widely dispersed sinkhole communities? How might rapidly rising surface temperatures affect the currently stable groundwater temperature regime?
Groundwater flow into the sinkholes is fueled by hydrostatic pressure from rainfall over land. But what are the current rates of groundwater flows to the sinkholes, and what might happen to the composition of the mats if flow rates change? Anthropogenic warming is likely to amplify the water cycle and make it more variable. How might precipitation changes affect life in the sinkholes over the short and long terms?
Clearly, many unknowns exist regarding how microbial mats will respond to human disturbances and climate change in the future. As we work to reveal these unknowns, mat ecosystems provide fascinating, dynamic, and biodiverse theaters of life for exploration.
Acknowledgments
The work described in this article was supported by a National Science Foundation grant (OCE2046958), a NOAA–University of Michigan Cooperative Institute for Great Lakes Research grant (NA22OAR4320150), a NASA–Michigan Space Grant Consortium grant (80NSSC20M0124), and shipboard and dive support from NOAA’s Thunder Bay National Marine Sanctuary.
References
Biddanda, B. A., et al. (2012), Rock, water, microbes: Underwater sinkholes in Lake Huron are habitats for ancient microbial life, Nat. Educ. Knowl., 3(10), 13, nature.com/scitable/knowledge/library/rock-water-microbes-underwater-sinkholes-in-lake-25851285/.
Biddanda, B. A., et al. (2015), Seeking sunlight: Rapid phototactic motility of filamentous mat-forming cyanobacteria optimize photosynthesis and enhance carbon burial in Lake Huron’s submerged sinkholes, Front. Microbiol., 6, 930, https://doi.org/10.3389/fmicb.2015.00930.
Biddanda, B. A., et al. (2021), Extant Earthly microbial mats and microbialites as models for exploration of life in extraterrestrial mat worlds, Life, 11(9), 883, https://doi.org/10.3390/life11090883.
Biddanda, B. A., A. D. Weinke, and I. P. Stone (2023), Extant mat microbes synchronize vertical migration to a diel tempo, J. Great Lakes Res., 49, 220–228, https://doi.org/10.1016/j.jglr.2022.10.006.
Grim, S. L., et al. (2021), Omics-inferred partitioning and expression of diverse biogeochemical functions in a low-O2 cyanobacterial mat community, mSystems, 6(6), e01042-21, https://doi.org/10.1128/mSystems.01042-21.
Hebting, Y., et al. (2006), Biomarker evidence for a major preservation pathway of sedimentary organic carbon, Science, 312(5780), 1,627–1,631, https://doi.org/10.1126/science.1126372.
Klatt, J. M., et al. (2021), Possible link between Earth’s rotation rate and oxygenation, Nat. Geosci., 14, 564–570, https://doi.org/10.1038/s41561-021-00784-3.
McGovern, C. A., et al. (2023), Unbiased analyses of ITS folding motifs in a taxonomically confusing lineage: Anagnostidinema visiae sp. nov. (cyanobacteria), J. Phycol., 59, 619–634, https://doi.org/10.1111/jpy.13337.
Nold, S. C., et al. (2013), Underwater sinkhole sediments sequester Lake Huron’s carbon, Biogeochemistry, 115, 235–250, https://doi.org/10.1007/s10533-013-9830-8.
Voorhies, A. A., et al. (2012), Cyanobacterial life at low O2: Community genomics and function reveal metabolic versatility and extremely low diversity in a Great Lakes sinkhole mat, Geobiology, 10, 250–267, https://doi.org/10.1111/j.1472-4669.2012.00322.x.
Voorhies, A. A., et al. (2015), Ecological and genetic interactions between cyanobacteria and viruses in a low-oxygen mat community inferred through metagenomics and metatranscriptomics, Environ. Microbiol., 18, 358–371, https://doi.org/10.1111/1462-2920.12756.
Author Information
Bopaiah A. Biddanda ([email protected]) and Anthony D. Weinke, Robert B. Annis Water Resources Institute, Grand Valley State University, Muskegon, Mich.; Ian P. Stone, School for Environment and Sustainability, University of Michigan, Ann Arbor; Steven A. Ruberg, NOAA Great Lakes Environmental Research Laboratory, Ann Arbor, Mich.; and Phil A. Hartmeyer, NOAA Thunder Bay National Marine Sanctuary, Alpena, Mich.; now at NOAA Ocean Exploration and University Corporation for Atmospheric Research, Alpena, Mich.


