Editors’ Vox is a blog from AGU’s Publications Department.
The surface of Mars represents the comprehensive geochemical inventory of the interactions between the lithosphere, atmosphere, and/or hydrosphere over a period of more than four billion years. By investigating the chemical composition and variability of surface materials, we can reconstruct the planet’s evolutionary history and investigate how different geological processes shaped the surface environment of Mars over geologic time. Due to their unique properties and global distribution, reactive salts of chlorine, called oxychlorine species, constitute an important component of the Martian surface.
A new article in Reviews of Geophysics investigates the state of the knowledge and discusses potential areas of future exploration for oxychlorine species on Mars. Here, we asked the author to give an overview of oxychlorine species on Mars, how scientists study them, and what questions remain.
Why is it important to understand the composition of the surface environment of Mars?
Certain surface materials can serve as diagnostic indicators of early and contemporary aqueous activity on the Martian surface.
Certain surface materials—such as salts and hydrated minerals—can serve as diagnostic indicators of early and contemporary aqueous activity on the Martian surface. Accurately understanding the formation, evolution, and preservation of these minerals that formed in aqueous systems can provide crucial constraints on the chemistry and availability of water that are needed to evaluate habitability conditions on Mars. Furthermore, characterizing the modern surface composition is the essential first step in deconvoluting geochemical cycles as well as assessing regolith toxicity, important for future robotic, sample return, and human missions to Mars.
In simple terms, what are oxychlorine species and where have they been found on Mars?
Oxychlorine species are chemical compounds composed of chlorine and oxygen, ranging from stable salts like perchlorate and chlorate to reactive gases and transient intermediates. This diversity arises from the multiple oxidation states of chlorine, which vary from -1 in chloride (Cl-) to +7 in perchlorate (ClO4-). While perchlorate and chlorate (ClO3-) have been identified on Mars, highly reactive intermediates are also likely to exist, at least transiently, during oxychlorine formation and destruction processes.
These compounds are widely distributed across the Martian surface. The Phoenix lander first detected them in the northern plains, while the Curiosity and Perseverance rovers have confirmed their presence in soil, sediment, and rock samples within the Gale and Jezero craters, respectively. Furthermore, oxychlorine salts have been identified as inclusions within pristine Martian meteorites. These widespread detections suggest that oxychlorines are a global component of the Martian regolith, influencing the planet’s geochemical and environmental evolution.
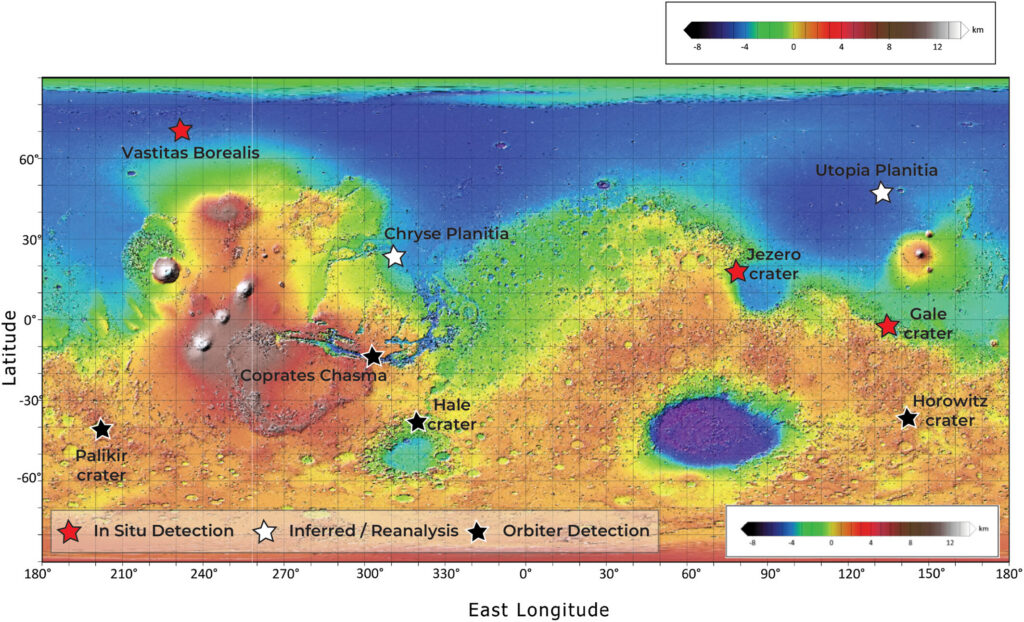
How do scientists detect and sample oxychlorine species?
Scientists have successfully employed various analytical techniques to identify oxychlorine species on the surface of Mars. The Phoenix lander used ion selective electrodes in the Wet Chemistry Laboratory (WCL) to detect perchlorate anions in the Martian regolith. Additional measurements from the Thermal and Evolved Gas Analyzer (TEGA) and the Surface Stereo Imager (SSI) also confirmed the presence of perchlorate anions. At Gale Crater, the Curiosity rover’s Sample Analysis at Mars (SAM) instrument identified these species by heating samples and measuring the evolution of oxygen and chlorine-bearing gases, such as HCl.
More recently, the Perseverance rover used its Raman and X-ray fluorescence spectroscopy instruments—SHERLOC, SuperCam, and PIXL— to detect oxychlorine species within altered rock assemblages at Jezero Crater. Beyond in situ analysis, orbital instruments like CRISM can be used to detect hydrated oxychlorine salts using visible and near-infrared spectroscopy. Finally, multiple analytical methods in terrestrial laboratories can detect oxychlorine species using spectroscopy, chromatography, and diffraction techniques.
What are recent advances in our understanding of oxychlorine formation and destruction on Mars?
Early research focused on atmospheric production, but the low abundance of oxygen-bearing gases in the Martian atmosphere failed to explain the high concentrations of perchlorate on Mars. Recent studies have identified three additional formation mechanisms: plasma redox chemistry during electrostatic discharges, heterogeneous reactions between chlorine-bearing salts and energetic radiation, and aqueous processes. Among these, the irradiation of chloride minerals and ices by ultraviolet light or galactic cosmic rays is particularly effective on contemporary Mars because the thin atmosphere allows radiation to interact directly with the surface.
Regarding destruction, perchlorate salts can degrade into chlorate when exposed to galactic cosmic radiation. Furthermore, chlorate can be effectively consumed by dissolved ferrous iron or ferrous minerals at temperatures as low as 273 K. While perchlorate remains kinetically stable in the presence of most redox-sensitive materials, reactive intermediates like hypochlorite (ClO–) and ClO2 gas readily react with organic compounds, leading to their mutual destruction.
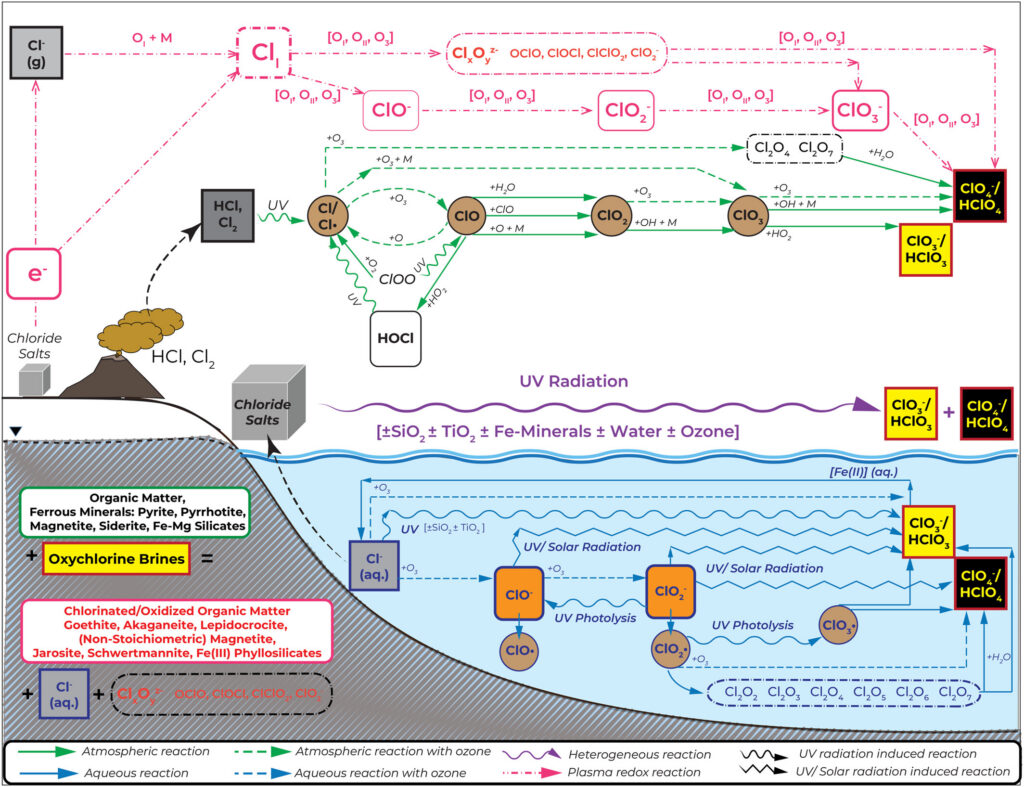
What does the presence of oxychlorine tell us about Mars’ history?
Oxychlorine species record the unique environmental history of Mars. Chlorine isotope data and detections in meteorites, such as Tissint and EETA79001, suggest an active oxychlorine cycle spanning 4 billion years, indicating that oxidizing fluids have been widespread throughout Martian history. Unlike Earth, where the nitrate-to-perchlorate ratio is high (~104), the ratio on Mars is less than one, except for inclusion in EETA79001. This discrepancy highlights fundamentally different geochemical fixation processes and nitrogen-chlorine cycles between the two planets.
Furthermore, chlorates are effective iron oxidants under Mars-relevant conditions and likely contribute to the formation of the planet’s ubiquitous ferric minerals. Additionally, as potent freezing point depressants, these salts may stabilize transient liquid brines even in modern equatorial regions. As a halogen-rich planet, Mars hosts a reactive surface chemistry where oxychlorine species play a substantially more dominant role than they do on Earth.
Is the presence of oxychlorine species helpful or harmful to human exploration and possible use of Mars?
Oxychlorine species can act as a potential hazard as well as a critical in situ resource for future human exploration.
Oxychlorine species can act as a potential hazard as well as a critical in situ resource for future human exploration. Perchlorate and chlorate salts can thermally decompose to release molecular oxygen (O2) and can thus potentially be used for human consumption. Approximately 60 kg of the Martian regolith, containing ~0.5 to 1 wt.% oxychlorine salt, could theoretically provide a single person’s daily oxygen supply. On the other hand, perchlorate is a well-known contaminant in drinking water since it interferes with thyroid functioning and can cause a goiter. Therefore, perchlorate in the Martian regolith could be a possible source of contamination for drinking water or agricultural systems. Owing to high chemical reactivity and oxidation potential, oxychlorine salts present in the Martian regolith are likely to pose persistent cleaning challenges for habitats, suits, and equipment during extra vehicular activity (EVA) on Mars. Additionally, agriculture in the oxychlorine-laden regolith might lead to contamination of plants and vegetables and could eventually lead to biomagnification in humans.
What are some of the remaining questions where additional research is needed?
While oxychlorine research has flourished over the last two decades, critical gaps remain regarding the spatial distribution and formation rates of distinct species. Recent detections of atmospheric HCl and electrostatic discharges necessitate a rigorous re-evaluation of Martian atmospheric chemistry. By leveraging emerging terrestrial models of chlorate formation, new pathways for Martian oxychlorine production can be proposed. Determining the relative contributions of atmospheric, plasma redox, and heterogeneous pathways is vital to understanding the evolution of the chlorine cycle and estimating equilibrium concentrations and residence times.
Furthermore, the chemical reactivity of transient intermediates, specifically ClO2 gas and chlorite, remains poorly understood regarding organic preservation at low temperatures. We also require precise thermodynamic data on complex salt mixtures to accurately predict brine stability. Ultimately, experimental validation of these salts as a viable in situ resource for oxygen and fuel is imperative for future human exploration and the interpretation of returned Martian samples.
—Kaushik Mitra ([email protected]; ![]() 0000-0001-9673-1032), The University of Texas at San Antonio, United States
0000-0001-9673-1032), The University of Texas at San Antonio, United States

