During the immense span of time that was the Precambrian—the first 88% of Earth’s 4.6-billion-year history—the planet witnessed milestone events like the dawn of life, the atmosphere’s oxygenation, and global glaciations that helped shape the world in which humanity exists today.
To better understand such momentous processes, scientists study Precambrian sedimentary rocks that have long endured the travails of tectonics and attempted erasure by erosion. For example, they study marine shales and limestones that record the chemistry of the water column. Because gases dissolved in seawater and those in the atmosphere interact where air meets sea, understanding the geochemistry in Precambrian marine shales and limestones lets scientists tease out clues about bygone climes.
The unique swings in geochemistry observed in Precambrian rocks are much larger in magnitude than those that occurred in more recent and familiar periods in Earth’s history, said Alan Rooney, an Earth and planetary sciences professor at Yale University. The sedimentary strata also host evidence of the evolution of complex life, from single-celled organisms to multicellular eukaryotes. “Biologically, a lot is going on” in these rocks, he said.
However, to understand cause and effect when studying a Precambrian rock, “you need to know how old it is,” said Kaarel Mänd, a research fellow at the University of Tartu in Estonia. “Otherwise, you cannot place it in the sequence of events.”
To this end, geochronologists—scientists who tell time in the geologic record by measuring radioactive elements stored in rocks—are now applying innovative instruments to rejuvenate isotopic dating systems that had fallen out of fashion because of their often cumbersome analytical requirements, including large sample sizes and arduous preparation and measurement. In particular, these advances help geochronologists rapidly collect data for isochron diagrams, which first revolutionized the field more than 60 years ago by providing a way to determine the ages of otherwise inscrutable ancient rocks.
Precambrian Predicaments
Evidence for single-celled life exists as far back as the Archean eon. But the evolution of a specific type of single-celled life, cyanobacteria, likely played a key role in dramatically altering Earth’s atmosphere during the Great Oxidation Event between about 2.4 billion and 2.0 billion years ago. During this time, the atmospheric chemistry at Earth’s surface shifted from reducing conditions, in which oxygen is rapidly consumed, to oxidizing conditions replete with the gas. The Great Oxidation Event is “perhaps the most conspicuous big event that happened in the Precambrian,” said Mänd.
This long-term process set the stage for the evolution of eukaryotes—organisms that encase DNA within their cellular nuclei—which eventually began to breathe oxygen and grow into bigger organisms, said Annie Bauer, an assistant professor of geoscience at the University of Wisconsin–Madison. Whether this happened roughly simultaneously across the globe or in geographically isolated pockets at different times is still being studied. By comparing the timing of oxygenation from place to place, she said, scientists can determine whether these first whiffs arose together as a globally synchronous exhalation or as discrete puffs.
Later, from about 1.8 billion to 0.8 billion years ago, atmospheric oxygen levels flattened out and stabilized, leading some scientists to dub this time the “boring billion.” Yet multicellular life emerged during this time; important ores like copper, iron, lead, and zinc—sensitive to the amount of oxygen near them—were deposited; and continents such as ancient North America grew as supercontinents assembled.
The remainder of the geochemistry tucked into the Precambrian’s rock record features evidence of unusual climate dynamics perhaps related to Earth’s carbon cycle, said Marjorie Cantine, a postdoctoral fellow at Goethe University Frankfurt in Germany. Understanding the Earth system changes that might have led to the flowering of diverse, complex life during the Phanerozoic—the present geological eon—requires dating the rocks that hold these clues, she said.
“The biostratigraphy that helps geologists sort through time in the Phanerozoic is largely unavailable in Precambrian rocks.”
The Phanerozoic “[has] this really rich fossil record that you can use to tell time,” said Cantine. In contrast, Precambrian life was not mineralized. The biostratigraphy that helps geologists sort through time in the Phanerozoic is largely unavailable in Precambrian rocks, she said.
Scientists who delve into Precambrian rocks often rely on the physical position of different rocks in relation to one another to tell their relative ages, said Mänd. Once-molten magma, for instance, will always be younger than any sedimentary rock it cuts across. For that reason, dating that crosscutting igneous rock provides a minimum age for the sedimentary strata, although the sedimentary rock could still be many millions of years older than that minimum age, he explained.
Even in cases where scientists have tried to directly date marine Precambrian rocks, for example, they sometimes know the rocks’ ages only to within hundreds of millions of years, said Nick Roberts, a research scientist at the British Geological Survey.
Mathematical Tricks for Dating Rocks
Since Marie Curie first coined the term “radioactivity” in the late 1800s, the field of radiometric dating of rocks—geochronology—has emerged and matured.
Naturally occurring elements have different isotopes, in which the number of protons is the same but the number of neutrons varies, resulting in different masses of the same element. For some elements, certain isotopes are radiogenic, meaning they exist because of radioactive decay. Geochronology focuses on measuring the decay of a radioactive “parent” isotope to a radiogenic “daughter” one, like the decay of certain isotopes of uranium to lead or rubidium to strontium. “We know pretty well how quickly that happens,” said Cantine. By measuring parent-daughter ratios in a rock or mineral, scientists can calculate when the dated material came into existence. “We’re able to do that extraordinarily well in certain special minerals” that form with parent isotopes but without any daughter products, she said.
One of those special minerals is zircon. A hardy mineral made from zirconium, silicon, and oxygen, zircon crystals can retain their initial geochemical signatures despite being bathed in magma or doused in water. Crucially for geochronologists, zircon crystals readily incorporate uranium as they form—when the clock starts, so to speak—but they do not initially incorporate daughter isotopes of lead. Any lead found in zircon today, said Cantine, is there solely because of radioactive decay over time.
Measuring uranium and lead in zircons can sometimes help geochronologists when they’re able to find these time capsules. For example, layers of ash belched by volcanoes often contain zircons that effectively date the time of eruption. Such layers can provide markers in successions of marine rocks, which often lack any other indicator of time. Modern laboratory techniques enable the development of very high precision dates from zircon crystals, making it so that finding zircon-bearing ashes is the dream scenario for Precambrian geologists. Unfortunately, volcanic ashes do not occur everywhere Earth scientists seek geochronology on sedimentary rocks, said Cantine. In another approach, geochronologists date many tens, or even hundreds, of zircons extracted from sandstones. Known as detrital zircons, these minerals initially formed in other rocks that were then eroded and redeposited—sometimes multiple times—before arriving at their terminal sedimentary destination. Detrital zircons can help fingerprint the source regions of the sands in a sandstone and provide a maximum age constraint for the rock, said Bauer, but they can’t tell you the actual time at which the rock formed. Recent research used a high number of detrital zircons dated at low precision using quick laser methods and then dated the youngest of those grains with more time-consuming high-precision methods. Although this process can sometimes get close to the formation age, it will still be a maximum age.
Luckily, geochronologists have several other radiometric systems at their disposal to directly date marine sedimentary rocks like shales and carbonates. Unfortunately, as shales and carbonates form, they incorporate various radiogenic daughter products in addition to parent isotopes, meaning a single measurement is likely to yield an erroneously old age for a rock.
To overcome such limitations, though, “we’re able to do some mathematical tricks by measuring multiple different locations within the same rock,” said Cantine.
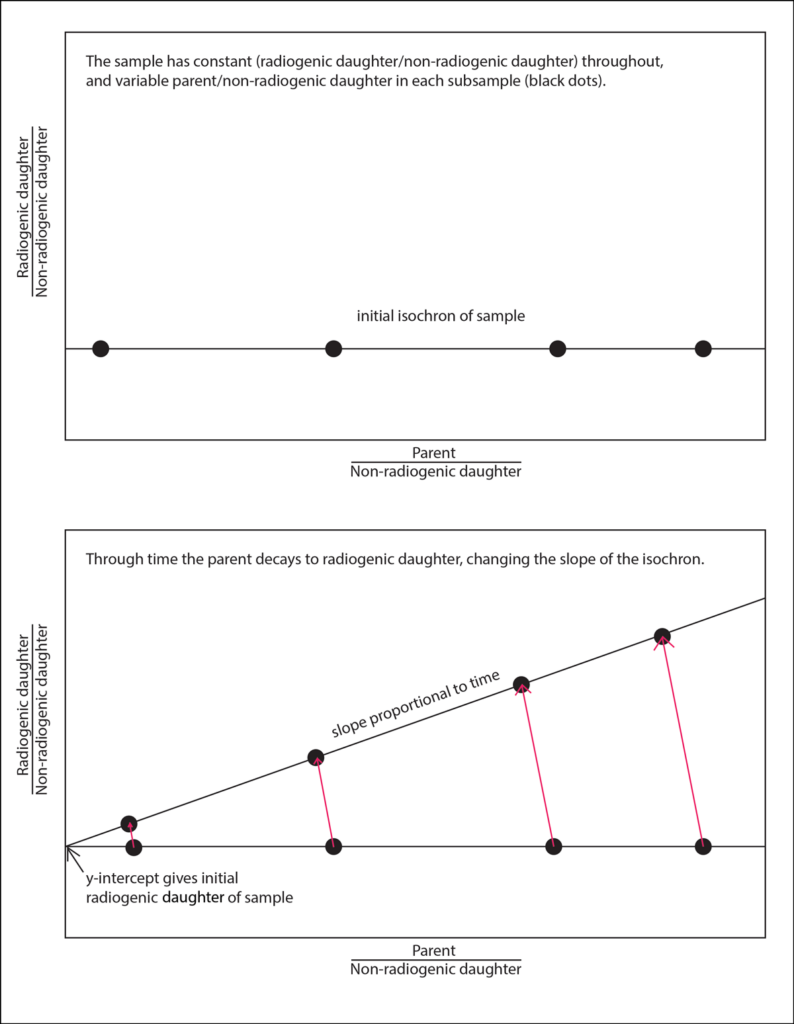
The ultimate trick is the isochron method, first conceived in 1961, which requires no knowledge of how much radiogenic daughter isotope a sample incorporated at the time it formed. A “device of magnificent power and simplicity,” wrote Brent Dalrymple in The Age of the Earth in 1991, “an isochron is a line of equal time.” Obtained by analyzing several minerals from the same rock or several rocks that formed together but that contain different amounts of the parent element, the simplest form of an isochron requires measurement of only a parent, its radiogenic daughter product, and a third quantity—the relative amount of a nonradiogenic isotope of the daughter element, which should remain constant over the lifetime of a sample. The inherent assumption, said Cantine, is that the rocks or minerals in question began with the same amount of all isotopes of the daughter, regardless of whether they were produced by radioactive decay, and that no later process has perturbed that balance.
By dividing both the amount of the parent and the amount of the radiogenic daughter by the amount of that third quantity—a nonradiogenic daughter isotope—and then plotting the resulting values on the x and y axes, respectively, wrote Dalrymple, “the points will fall on a line whose slope is a function of the age of the rock.” In other words, simple division allows geochronologists to exploit the equation of a line.
Resuscitating an Old Method
Continental rocks exposed to water and weather at Earth’s surface deteriorate into smaller bits, including clay minerals, through physical and chemical erosion. When these flecks of former rocks end up in the seas, they eventually form layers of fine-grained sedimentary rocks called shales. That’s how shales have formed for more than 3 billion years, Mänd said.
Often rich in organic matter, these thinly layered rocks often form in deep ocean waters. One way to date shales as old as about 2.5 billion years, said Rooney, is by using the decay of a radiogenic isotope of rhenium, a metal, to another metal, osmium. But the process of isolating rhenium from osmium is arduous, involving several days of complicated laboratory work. Dates developed through such arduous research are playing increasingly important roles in telling time in ancient sedimentary rocks lacking zircon-bearing ashes.
When rhenium decays to osmium, it does so via a process called beta decay, in which an atom loses or gains a proton (i.e., the daughter becomes a different element) but has the same mass as the parent (i.e., the two have the same combined total number of protons and neutrons). This process holds true for any beta decay system, including rubidium’s transformation to strontium, said Mikael Tillberg, a postdoctoral fellow at Linnaeus University and the University of Gothenburg, both in Sweden. The isochron method was first demonstrated using the rubidium-strontium dating system, but other methods that often proved faster or cheaper to employ partially supplanted its use. As such, said Tillberg, rubidium-strontium dating is often viewed as antiquated. However, innovative technologies are reinvigorating this vintage timepiece that can constrain the finicky ages of those fine-grained shales.
Laser ablation systems let geochronologists shoot holes on the scale of tens of micrometers in target materials, said Tillberg, dramatically reducing the sample size needed per measurement. The laser ablates the target rock, turning it into an aerosol that is immediately piped to a mass spectrometer.
Because the parent and daughter isotopes used in rubidium-strontium geochronology have the same mass, attempting to measure them simultaneously in an instrument designed to measure different masses may seem counterintuitive. A triple quadrupole mass spectrometer solves this quandary, said Tillberg. (A quadrupole in this context consists of four parallel rods, with each opposing pair having a different voltage that attracts or repels charged particles.)
When ablated, aerosolized rock enters the instrument, and a plasma ionizes it into charged particles. Then, the first quadrupole separates the particles according to mass, explained Tillberg. The next quadrupole contains a gas like nitrous oxide, which donates oxygen to strontium but not to rubidium. The strontium, now combined with oxygen, has a higher mass that is easily separated from rubidium by the third quadrupole, he said. This potent combination of laser ablation and triple quadrupole mass spectrometry allows both isotopes to be measured from the same imperceptibly small slug of sample while eliminating the complicated and time-consuming laboratory work needed to dissolve a rock and physically separate parent and daughter isotopes.
Laser ablation preserves samples for future reference.
“Honing into a single layer in the rock record…especially if samples come from drill cores that already have small sample sizes,” becomes much simpler with this updated method, said Darwinaji Subarkah, a doctoral student at the University of Adelaide in Australia. Because the measurement process is so fast, multiple spots from a single sliver of sample can be ablated and analyzed in hours, generating the data necessary for an isochron, he said. Furthermore, whereas traditional rubidium-strontium methods consume entire samples, laser ablation preserves the sample, leaving the measured rock available for future reference, said Subarkah. Moreover, because laser ablation requires much smaller amounts of material, additional sample is often available for analysis by other methods.
However, to generate a robust isochron, the analyzed parts of a rock must be texturally equivalent. “You need to be able to assume that your initial strontium composition of all the [sample] was the same,” said Bauer. “That’s what makes sedimentary rocks really tricky.”
Careful petrographic characterization, especially at the nanoscale, can potentially solve this problem, helping to differentiate among clays that came from eroding continents, clays that grew as the sediment became a rock, and clays that changed as the rock warmed and recrystallized, said Subarkah. By combining petrographic analyses with laser ablation, he said, “we’re actually looking at individual relationships between the different mineral phases.”
Carbonate Conundrums
Phanerozoic carbonate rocks—limestones and dolomites—are “often completely composed of these big pieces of fossil [animals] stuck together,” Mänd said, which helps tell time.
But carbonates exist from more than 3 billion years ago, well into the Precambrian, when they were “completely built, as far as we know, by microbes,” according to Cantine.
Sedimentary carbonates can be dated using uranium’s decay to lead. But carbonates don’t incorporate much uranium, and they tend to include lead as they form, said Cantine. “That means that we have problems on both the parent and the daughter side.” A variation on the simple isochron, along with lasers, has rejuvenated uranium-lead dating for carbonates.
The first attempt at uranium-lead carbonate geochronology began in the 1980s, said Roberts, and continued through the late 1990s with studies of Precambrian rocks, mostly. Early papers describe methods that involved drilling carbonate rock samples, dissolving chunks with acids, chemically separating the uranium and lead, and making measurements on a thermal ionization mass spectrometer (TIMS) instrument, Roberts explained. This required big samples, a lot of time in the lab, and expensive equipment. However, carbonates are notoriously complicated at relatively small spatial scales, and by dissolving a large piece for analysis, any variations of uranium or lead within individual crystals or across the sample are lost as they are averaged into a single data point, he said.
“Carbonate rocks are wonderfully sensitive to the environment around them.”
Because geochronologists can focus their lasers to zap rocks at a scale of tens of micrometers, many measurements can be obtained rapidly from a single sample, allowing researchers to observe small-scale variations. These previously inscrutable variations provide the spread in measurements needed for a good isochron, said Roberts. Although individual measurements obtained by laser ablation come with higher uncertainties than data collected by traditional methods, the sheer number of measurements made possible by using lasers means that isochron-determined ages also can be precise.
Carbonates “are wonderfully sensitive to the environment around them,” said Cantine. Carbonate rocks can record temporally distinct processes, such as the initial deposition or precipitation of carbonate, its transformation into rock, any subsequent deformation by burial or tectonic processes, and even uplift from the ocean floor to the tops of mountains. Within a single sample, she said, “you could potentially have multiple meaningful ages preserved.” Because these different processes often leave behind texturally distinct carbonates visible only under the microscope, combining petrographic examination with laser ablation techniques is critical for connecting a date to a specific geological process, she said.
Nevertheless, just because we have lasers doesn’t mean it’s time to leave the old methods in the past. TIMS measurements in particular are highly precise, said Cantine. In her work, she’s aiming for the best of both worlds, she said, by rapidly assessing carbonate dates using laser ablation and then following up with TIMS analyses to confirm the results.
What Came First?
By precisely dating sedimentary rocks with updated geochronologic techniques, said Mänd, scientists can begin to solve some long-standing chicken-and-egg problems in Precambrian geology. For example, snowball Earth glacial events recorded in sedimentary rocks that happened about 2.4 billion and 0.6 billion years ago coincide with both atmospheric oxygen fluctuations and peculiarly large swings in Earth’s carbon chemistry.
The older snowball Earth event (the Huronian glaciations) may have been triggered by excess oxygen produced by cyanobacteria. But the widely accepted age constraints for this event come from 2.45-billion-year-old Archean rock that sits below the sedimentary rocks recording the past global freezes, along with a 2.22-billion-year-old crosscutting igneous intrusion into the sedimentary rocks, leaving a span of nearly 300 million years, said Bauer. With better time constraints, scientists parse just how many glaciations occurred, whether they were truly global, and what their relationship is to the cyanobacteria-fueled oxygen spike and the carbon swings recorded in these rocks, she explained.
During and after the younger snowball Earth events during the Cryogenian period, Earth’s earliest animals evolved amid continued episodic glaciations and more curious carbon records. But as in the Huronian, the relations and timing of these glaciations, carbon fluctuations, and evolution of life are unclear. Understanding the time component with the help of the best available geochronologic systems and instrumentation, said Cantine, “is critical for figuring out how and in what ways these events might be connected.”
—Alka Tripathy-Lang (@DrAlkaTrip), Science Writer


