We know that water lurks within Martian soil. But in what form?
According to measurements by Mars landers and rovers, Martian soil may store water in clays, unusual silica such as opal, and hydrous sulfates such as gypsum and even as ground ice. Hydrous sulfates, also found on Earth, tantalize researchers because they offer vital clues to the nature of soil habitability. Characteristics of this habitability include the acidity and concentration of brines that may occur, with possible relevance to seasonally observed flow features of soil within southern craters.
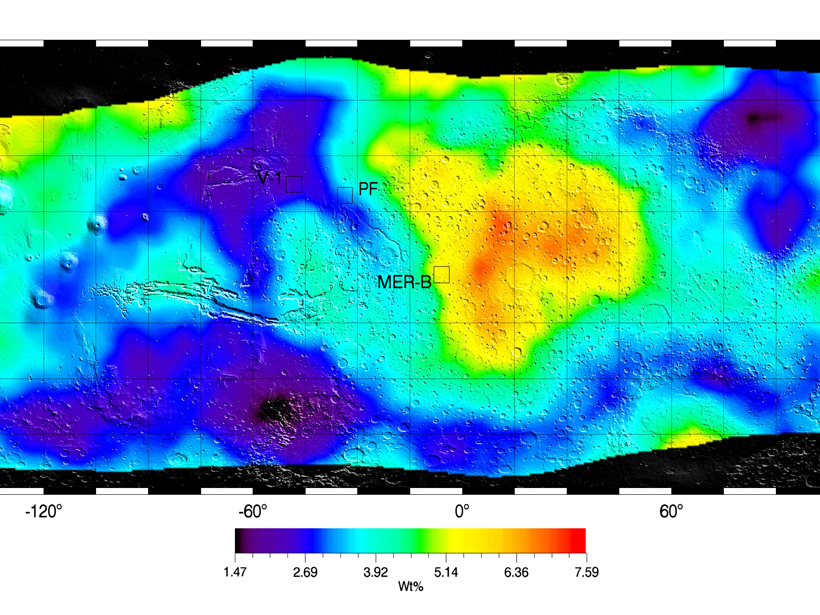
Data supporting the widespread presence of hydrous sulfates have been geographically limited: rovers and landers do not travel far. A new study widens the view. Taking advantage of years of data from the 2001 Mars Odyssey orbiter, Karunatillake et al. present maps of hydrogen and sulfur that may reveal the importance of sulfates to storing water in the soil of southern Mars
The orbiter’s gamma ray spectrometer senses the energy and number of photons—far more energetic than X-rays—coming from the Martian surface and thus the relative amounts of various periodic table elements needed to generate them. Analyzing peaks in the spectroscopic data that indicated the presence of sulfur and hydrogen, the authors estimate the molar ratio of water to sulfur to be between 2.4 and 4.0 for the majority of the planet’s southern hemisphere, at depths approaching half a meter.
Meanwhile, the spatial association between hydrogen and sulfur at these regional scales suggests the possibility of sulfates binding water in bulk soil. Secondarily, the ratios are consistent with those expected for many iron sulfates. Alternatively, mixtures of calcium and magnesium sulfates could also yield the signature.
Hydrous sulfates on Earth can form in brine pools as they desiccate. Iron sulfates in particular can also allow brines to exist at temperatures much lower than those of many chlorides. Thus, the widespread possibility of the hydrated minerals suggests that sometime in Mars’s past, brines may have diffused through its bulk soil in processes that parallel the efflorescence that forms white fuzzy crystalline deposits in many a basement wall. Volcanic exhalations may have also contributed to the pervasiveness of hydrous soil sulfates.
Further, with the presence of such activity come the questions of a still-active water cycle, of fleeting pockets of brines across the Martian seasons, of a shallow reservoir of extractable water for human explorers, and maybe even of habitability for the hardiest forms of life (Geophysical Research Letters, doi:10.1002/2014GL061136, 2014).
—Shannon Palus, Freelance Writer
Citation: Palus, S. (2015), Water beneath the surface of Mars, bound up in sulfates, Eos, 96, doi:10.1029/2015EO027799. Published on 14 April 2015.
Text © 2015. The authors. CC BY-NC 3.0
Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.