Earth has skin. Like our own, Earth’s is a living, porous membrane with multiple layers and a complex structure that is vital for survival. Unlike ours, Earth’s skin—also known as the critical zone—arises from biogeochemical interactions among rock, soil, plants, microbes, and fluids that continually reshape and rejuvenate it. The critical zone extends from the top of the vegetation canopy to tens or even hundreds of meters into the subsurface, supporting terrestrial ecosystems and regulating exchanges of water, dissolved solutes, and gases between the land, atmosphere, and hydrosphere. It also absorbs, stores, and releases carbon, water, and nutrients, with direct implications for climate and ecosystem health.
Along with the critical zone, Earth’s capacity to support life has evolved as a direct result of complex interactions between organisms and Earth materials. And evidence of life’s role in shaping Earth’s surface has been recorded in the sedimentary record. The rise of molecular oxygen roughly 2.4 billion years ago in the Great Oxidation Event, which was mediated by the proliferation of early phototrophic organisms similar to cyanobacteria, changed Earth’s atmosphere from a reducing to an oxidizing environment. This change caused many species to die out, but it also enabled the development of multicellular life, including, eventually, mammals. Meanwhile, oxidation of iron and manganese in Earth’s crust altered the structure, mineralogy, and geochemistry of surface materials, as exemplified in banded iron formations.
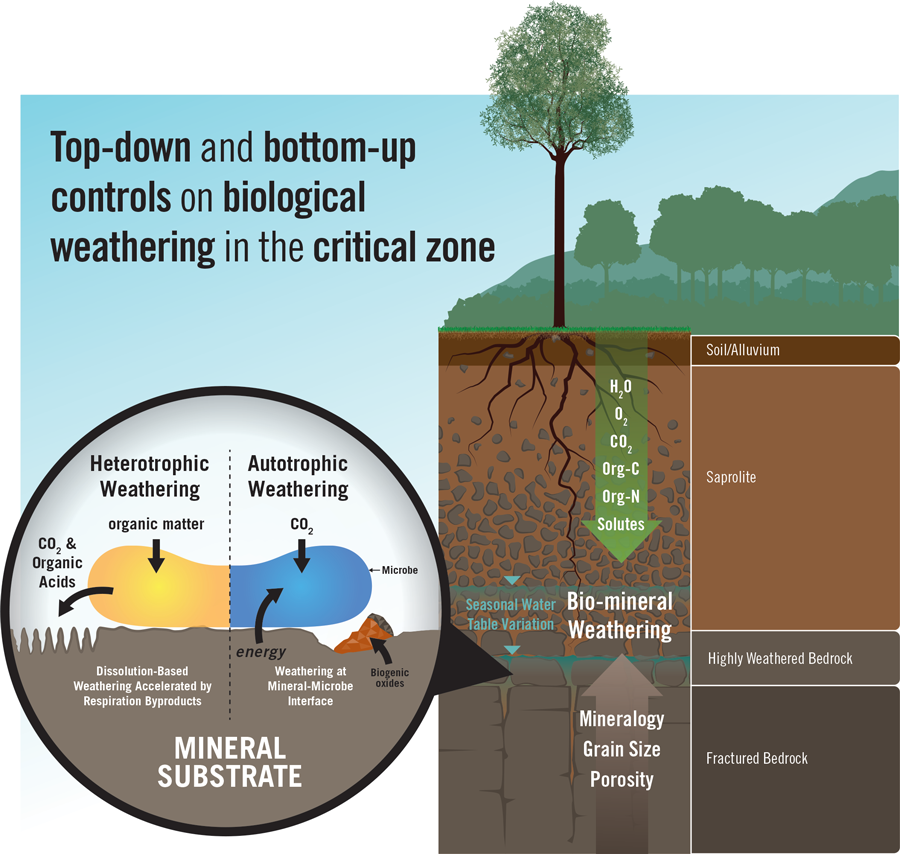
A grand challenge for critical zone scientists is to resolve the interactions and feedbacks between biota and Earth materials that give rise to critical zone structure and its life-supporting functions, as well as how the structure and these functions change with time, climate, and rock type. Research over the past decade combining real-time field and laboratory measurements with modeling and experimentation has helped us address this challenge and reveal that the diverse ways that the biosphere “breathes” directly influence the function and evolution of Earth’s living skin, even at great depths (Figure 1).
Teleconnections Speed Soil Formation
Pore spaces in soils and layers of fragmented bedrock, called regolith, underlying forested hillslopes teem with roots, microbes, dead biomass, and metabolites. Because of limitations on how fast gas can diffuse from these pores, they become highly enriched in carbon dioxide (CO2) resulting from plant and microbial respiration. It is generally thought that this CO2 diffuses upward, returning carbon captured during photosynthesis back to the atmosphere. By pairing vertically distributed CO2 and O2 gas sensors at different hillslope locations in the Catalina-Jemez Critical Zone Observatory (CZO) in Arizona and New Mexico, Sánchez-Cañete et al. [2018] found, however, that more than half of the CO2 produced by soil respiration is not returned directly to the atmosphere. Rather, this CO2 is dissolved in soil pore waters, creating carbonic acid, which is then transported deeper or laterally in the critical zone.
The breathing of biota in near-surface soils is teleconnected to deep subsurface weathering processes, where it plays an important role in accelerating the transformation of bedrock to soil.
Subsequent observations using CZO sensor networks revealed that when wetting fronts propagate into the CO2-enriched subsurface, which happens during rainfall and snowmelt events, the fronts are “charged” with carbonic acid and promote pulsed episodes of silicate rock (mica-bearing schist, specifically) dissolution and clay mineral formation along hydrologic flow paths that extend deeper than the bioactive soil zone [Olshansky et al., 2019]. In this way, the breathing of biota in near-surface soils is teleconnected to deep subsurface weathering processes, where it plays an important role in accelerating the transformation of bedrock to soil.
This work also highlighted that CO2 transported along deep subsurface hydrologic flow paths can emerge outside the measurement footprint of eddy covariance towers, which are used to quantify ecosystem respiration. This CO2 may return to the atmosphere only after CO2-enriched groundwater is discharged into streams and rivers, thereby effectively evading detection by the sensor towers designed to measure it. The coupling of biota to rock weathering through such transport of acidified water is consistent with an analytical model from Calabrese et al. [2017] that suggested that the hydrologic transport of carbonic acid from the bioactive rooting zone into the bedrock weathering zone may be as important as reactions occurring at mineral surfaces in determining chemical weathering rates.
In some cases, teleconnections between surface-colonizing ecosystems and the deep critical zone are more direct. For example, in a study at the Susquehanna Shale Hills CZO in Pennsylvania, Hasenmueller et al. [2016] found that roots and associated microbes extended deep into shale bedrock fractures. These investigators also observed that fractures lined with bioactive root tissue accumulated the same kinds of secondary minerals found in conjunction with roots in the soil zone. These results suggest that the CO2 production and microbial community proliferation stimulated locally by plant roots can also play active roles in the conversion of rock to regolith and soil.
Plant–Microbe Mediation
Plants transform solar energy into chemicals like carbohydrates, proteins, lignin, and low–molecular weight organic acids that are injected into the critical zone through root networks and that fuel diverse heterotrophic (organic matter–metabolizing) microbial communities. Microbes are found throughout the critical zone, but their functional roles and species biodiversity change with depth because of variations in habitat, including in the moisture dynamics and the availability of energy and carbon sources for growth [Akob and Küsel, 2011]. Key questions posed by critical zone scientists include, How deep do signals from surface plants extend into the critical zone, and how does this extension create depth-dependent trends in microbial communities? Addressing these questions is essential because microbes drive many biogeochemical cycles—not only of carbon and nitrogen but also of iron, manganese, silicon, and aluminum, for example—throughout the critical zone depth profile.
Prior studies of soil microbial ecology have focused on near-surface soil horizons predominantly, but critical zone scientists have delved much deeper.
Whereas prior studies of soil microbial ecology have focused on near-surface soil horizons, predominantly in the top 10 centimeters, critical zone scientists have delved much deeper to examine how microbial community composition and function change with depth and at different locations in complex hillslope terrain. Studying bacterial and archaeal composition as a function of depth in several excavated pits in the Boulder Creek CZO in Colorado, Eilers et al. [2012] found that microbial diversity decreased by 20%–40% between the surface and 2-meter depth, tracking exponential decreases in both microbial biomass and soil organic matter. Large variations in microbial composition were observed in different surface horizons across the Gordon Gulch watershed, but this lateral variation decreased significantly below the surface, such that deeper communities were much more similar irrespective of location.
The team found that the changes in microbial community composition that occurred between the surface and 2-meter depth at the Boulder Creek CZO exceeded those observed across a wide range of biome types sampled globally by the same team. That is, the microbial communities living just a meter or two below the surface were composed of very different strains than those living in the surface soils, and this difference was larger than those seen elsewhere worldwide. This observation highlights the heterogeneous nature of the critical zone profile, in which environmental conditions for life can change dramatically over very short vertical distances.
To determine whether these trends hold beyond Boulder Creek, Brewer et al. [2019] examined depth-dependent trends in bacterial and archaeal taxa to 1-meter depth in 20 soil profiles excavated in sites spanning the full national network of CZOs. This team also reported an average decrease in microbial diversity with depth and the associated shift from carbon- and nutrient-rich to carbon- and nutrient-poor conditions. However, several strains consistently increased in relative abundance with depth across multiple locations, especially Dormibacteraeota, whose genome provides the capacity to synthesize and store carbohydrates and the potential to use carbon monoxide as a supplemental energy source. Such capabilities are likely key to survival in the nutrient-deficient deep critical zone.
Deeper in the critical zone, the availability of organic matter as an energy and carbon source for heterotrophic microbial growth becomes progressively more limited. As a result, chemolithoautotrophy is expected to become increasingly important—as seen, for example, in fractured bedrock at much greater depths (kilometers) [Osburn et al., 2014]—but direct evidence of such an increase has been lacking. Chemolithoautotrophic organisms obtain their energy from reduced inorganic compounds (as opposed to organic matter) and their carbon for growth from CO2. Both heterotrophic and chemolithoautotrophic metabolisms affect mineral transformations and hence critical zone evolution, albeit differently.
Examining the previously hypothesized existence of a subsurface chemolithoautotrophic, iron-oxidizing microbial community at the bedrock interface tens of meters below the surface at the Luquillo CZO in Puerto Rico [Buss et al., 2005], researchers recently showed that bacteria can, indeed, obtain energy directly from the oxidation of ferrous iron (Fe2+) in granitic bedrock [Napieralski et al., 2019]. This iron-silicate bio-oxidation process resulted in precipitation of ferric (Fe3+) oxide nanoparticles, which accumulated within pores near the weathering front, where fresh bedrock is transformed into regolith. The microbially oxidized rock was then more susceptible to acid-promoted dissolution, like that which occurs when CO2-charged waters enter the deep critical zone (as discussed above). The results of this and an ongoing companion study suggest potential positive feedbacks between CO2 production mediated by interactions between plant roots and heterotrophic microbes in the near surface and chemolithotrophic mechanisms of mineral oxidation at greater depths.
Revealing Critical Zone Complexities
The evolution of critical zone architecture ultimately feeds back to support productive ecosystems and the services they provide to society.
Altogether, these and other results from CZOs have begun to reveal complex interactions among plants, microbes, and minerals that drive the weathering and structure of the critical zone. The evolution of critical zone architecture, particularly the biologically mediated transformation of bedrock to soil, ultimately feeds back to support productive ecosystems and the services they provide to society, like carbon and water storage, ecosystem nutrition, and the provision of food and fiber.
Further explorations of interactions between top-down and bottom-up controls on bedrock weathering, soil formation, and critical zone structure should address important outstanding questions such as how deep into the critical zone the weathering effects of plants extend and what influences rock type has on microbial colonization of the deep critical zone and associated bioweathering reactions. Such efforts will continue to uncover vital insights into how Earth’s remarkable skin works and how it affects those of us living on it.
References
Akob, D. M., and K. Küsel (2011), Where microbes meet rocks in the Earth’s critical zone, Biogeosciences, 8, 3,531–3,543, https://doi.org/10.5194/bg-8-3531-2011.
Brewer, T. E., et al. (2019), Ecological and genomic attributes of novel bacterial taxa that thrive in subsurface soil horizons, mBio, 10, e01318-19, https://doi.org/10.1128/mBio.01318-19.
Buss, H. L., et al. (2005), The coupling of biological iron cycling and mineral weathering during saprolite formation, Luquillo Mountains, Puerto Rico, Geobiology, 3, 247–260, https://doi.org/10.1111/j.1472-4669.2006.00058.x.
Calabrese, S., A. J. Parolari, and A. Porporato (2017), Hydrologic transport of dissolved inorganic carbon and its control on chemical weathering, J. Geophys. Res. Earth Surf., 122, 2,016–2,032, https://doi.org/10.1002/2017JF004346.
Eilers, K. G., et al. (2012), Digging deeper to find unique microbial communities: The strong effect of depth on the structure of bacterial and archaeal communities in soil, Soil Biol. Biochem., 50, 58–65, https://doi.org/10.1016/j.soilbio.2012.03.011.
Hasenmueller, E. A., et al. (2016), Weathering of rock to regolith: The activity of deep roots in bedrock fractures, Geoderma, 300, 11–31, https://doi.org/10.1016/j.geoderma.2017.03.020.
Napieralski, S. A., et al. (2019), Microbial chemolithotrophy mediates oxidative weathering of granitic bedrock, Proc. Natl. Acad. Sci. U. S. A., 116, 26,394–26,401, https://doi.org/10.1073/pnas.1909970117.
Olshansky, Y., et al. (2019), Soil fluid biogeochemical response to climatic events, J. Geophys. Res. Biogeosci., 124, 2,866–2,882, https://doi.org/10.1029/2019JG005216.
Osburn, M. R., et al. (2014), Chemolithotrophy in the continental deep subsurface: Sanford Underground Research Facility (SURF), USA, Front. Microbiol., 5, 610, https://doi.org/10.3389/fmicb.2014.00610.
Sánchez-Cañete, E. P., G. A. Barron-Gafford, and J. Chorover (2018), A considerable fraction of soil-respired CO2 is not emitted directly to the atmosphere, Sci. Rep., 8, 13518, https://doi.org/10.1038/s41598-018-29803-x.
Author Information
Jon Chorover ([email protected]), University of Arizona, Tucson; Emma Aronson, University of California, Riverside; Jennifer McIntosh, University of Arizona, Tucson; and Eric Roden, University of Wisconsin–Madison
Citation:
Chorover, J.,Aronson, E.,McIntosh, J., and Roden, E. (2020), Life teems below the surface, Eos, 101, . Published on 24 September 2020.
Text © 2020. The authors. CC BY-NC-ND 3.0
Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.


