Every year, soils across Thailand are baked hard and bone-dry for months under the unrelenting tropical Sun. But once the long, hot buildup to the monsoon season comes to a head and the rains arrive—typically in May or June—the landscape transforms in a matter of weeks into a patchwork of verdant wetlands.
With this transformation comes a flurry of activity, because many of these wetlands are, in fact, rice paddies ready to be planted. Workers wade through the fields, scattering seed or planting seedlings one by one in the butter soft soil. Months later, as long as these plants have had adequate water and nutrients, they’ll be harvested for their all-important grains.
Rice paddies in Thailand and elsewhere are vital economically and for food security, with rice being the third-most-grown cereal commodity globally. They are also part of the worldwide system of wetlands. From rivers and lakes to marshlands and intertidal flats, wetlands are important ecological and geochemical systems because of the ecosystem services they provide: biodiversity, natural pollution remediation, carbon sequestration, and protection against storm surges, to name a few.

Rice paddies are also of special interest to geochemists, because the regular seasonal pattern of flooding and drainage in many paddy fields makes them ideal natural laboratories in which to study soil biogeochemical processes. Indeed, in several locations in Thailand in 2021, scientists from the Swiss Federal Institute of Technology (ETH Zurich) and Kasetsart University in Bangkok joined the workers in the fields as the growing season began. The scientists were there to plant not rice, though, but iron minerals, with the purpose of testing a new method for investigating how cycles of wetting and drying contribute to these minerals’ reactivity in soil.
A Linchpin of Soil Functioning
Iron is a linchpin of chemical cycling in the environment.
Iron is usually among the most abundant elements in soils, and it is a linchpin of chemical cycling in the environment. It’s thus of critical interest to farmers concerned with the availability of nutrients to their plants, engineers determining risks posed by toxic elements in soil, and land managers wanting to understand soil carbon storage. For several reasons, iron more often than not significantly controls how soil nutrients and toxic elements behave, how carbon is stored, and how other chemical processes play out in soils.
One reason is that iron is reactive in a variety of environmental conditions. In contact with air, oxidized forms of iron—including the yellow-brown to orange oxyhydroxide minerals commonly recognized as rust—are most stable and abundant. When a soil is flooded and the flow of oxygen through it is restricted, some of these minerals may dissolve. This dissolution occurs because some microorganisms can use iron minerals as an alternative to oxygen in the reduction and oxidation (redox) reactions they rely on for energy. In other words, some microorganisms essentially breathe redox-active iron (see video below), reducing oxidized iron and converting it to other forms.
Iron is also very versatile and combines with many other chemical elements to form a wide variety of minerals such as oxyhydroxides, carbonates, phosphates, sulfides, hydroxysulfates, and others that can host trace elements in their structures. What’s more, iron mineral particles tend to be very small and have large surface areas that allow them to bind other compounds. This property also makes iron mineral particles susceptible to rapid change under evolving environmental conditions.
Following Mineral Transformations in Field Soils
Studying the behavior of iron in soils using measurement techniques that are not specific to iron is challenging. The relatively small signatures of iron minerals are often overshadowed by those of the much more abundant silicate minerals that make up the bulk of soil mass.
With techniques that do selectively detect iron in soil, such as synchrotron X-ray absorption spectroscopy, researchers can follow changes in the composition of a whole soil sample, but not changes in a single target mineral. And if the complexity of the surrounding soil is stripped away to perform simplified laboratory experiments focusing on iron minerals, the mineral transformations observed do not always reflect what happens in natural soils.

Iron minerals enriched in iron-57 and having precisely controlled properties can be synthesized in the laboratory, then mixed into soils to undergo reactions similar to those of natural iron minerals.
To overcome the challenge of tracing iron mineral transformations in soil, we developed a new approach using a stable isotope of iron to label synthetic minerals. Iron-57 occurs naturally, making up about 2.1% of the iron in soils and exhibiting the same chemical behavior as other naturally occurring iron isotopes (iron-54, 5.8%; iron-56, 91.8%; iron-58, 0.3%). Iron minerals enriched in iron-57 and having precisely controlled properties can be synthesized in the laboratory, then mixed into soils to undergo reactions similar to those of natural iron minerals. Even if the experimental enrichment of iron in the soil is small, the iron-57 is predominantly present in the synthetic minerals, allowing us to focus specifically on what happens to those minerals.
We chose iron-57 as a tracer in our experiments because of the ability to analyze it using Mössbauer spectroscopy. This technique, based on Rudolf Mössbauer’s fortuitous (and 1961 Nobel Prize–winning) discovery of recoilless nuclear resonance fluorescence, is sensitive to the redox state and chemical environment around iron-57 atoms in a sample. Crucially, all other isotopes of iron are invisible using this technique.
Mössbauer spectroscopy has been widely used in the Earth sciences, including for mineralogical analyses of soil samples and Martian rocks. In our application, adding iron-57 minerals into soils and tracing them with Mössbauer spectroscopy allows us to follow otherwise hidden mineral transformations (Figure 1).
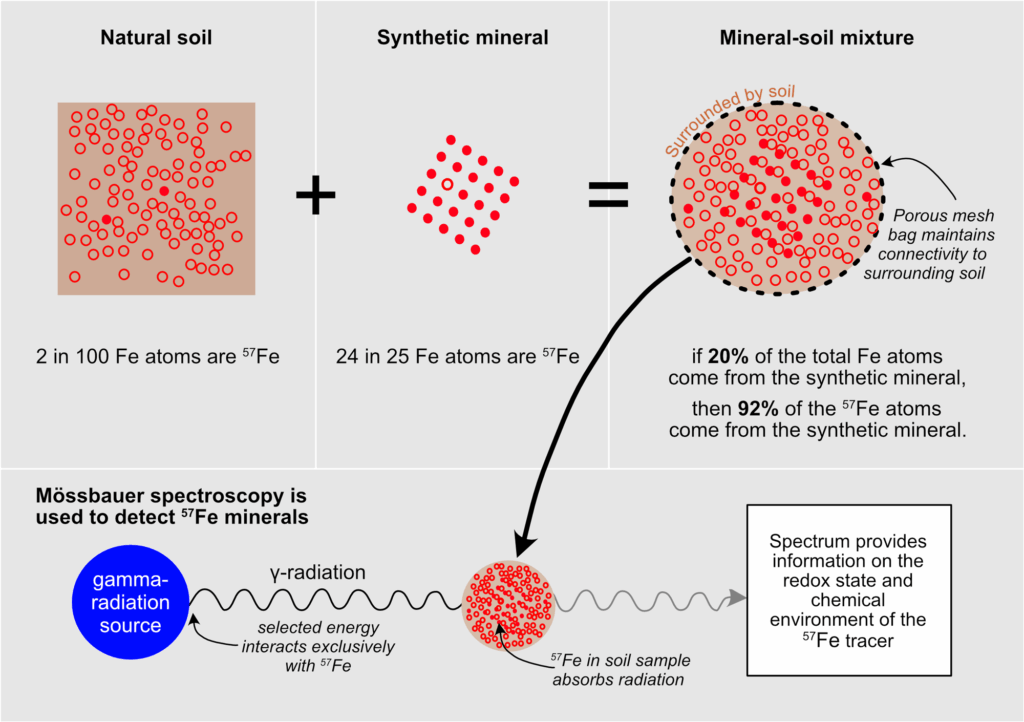
After adding portions of synthetic jarosite (a potassium-iron hydroxysulfate) or ferrihydrite or lepidocrocite (both iron oxyhydroxides) to small plots in the Thai rice paddies early in the growing season, we went back several times until the end of the season 4 months later to collect soil samples for analysis.
The mineral transformations we observed with Mössbauer spectroscopy were dominated by the dissolution of the added minerals and the release of reduced iron into soil pore water. Proximity to bacteria in the soil promoted mineral dissolution, and some of the released iron either remained dissolved or was trapped on soil particles. New minerals that formed—including green rust, a highly reactive hydroxide mineral that is usually difficult to detect in the environment—tended to be nanocrystalline in size and often contained both reduced and oxidized forms of iron.

Such results can shed light on biogeochemical cycling that affects ecosystem processes. Dissolution of iron minerals might lead to releases of associated pollutants, nutrients, or carbon compounds, for example. On the other hand, we observed that many of the reaction products in soil are nanocrystalline minerals, which have large reactive surface areas that might adsorb dissolved compounds such as metals.
We have also applied this new approach to understand a range of iron mineral transformation processes in soil and sediment environments other than rice paddies. In sediments along Germany’s north coast, for example, we observed the in situ formation of vivianite, a reduced iron phosphate mineral, in a matter of weeks. Phosphorus in vivianite has limited bioavailability, so formation of the mineral can control the availability of phosphorus in the environment and potentially reduce the risk of eutrophication. In addition, we have used the method to study oxidation reactions of reduced iron minerals, sulfidization of vivianite and lepidocrocite leading to the formation of iron sulfide minerals like greigite, and interactions between iron and organic matter during redox cycles.
An Array of Applications
Iron minerals are ubiquitous in natural environments and are used in many engineering applications. We anticipate that iron-57 labeling of minerals coupled with Mössbauer spectroscopy, although applied only to soils so far, could help to answer questions about transformations of these minerals in other domains of the Earth sciences and beyond.
Using iron-57 tracers could contribute to studies on the origins of iron mineral assemblages in sedimentary deposits on Earth or other astronomical bodies such as Mars.
Using iron-57 tracers could, for example, contribute to studies on geological processes, including weathering or metamorphism, or on the origins of iron mineral assemblages in paleosols and other sedimentary deposits on Earth or other astronomical bodies such as Mars.
Experiments with iron-57-labeled minerals could also help to understand redox-driven iron mineral transformation processes at work in applied geoscience technologies. In pollution management, for example, permeable reactive barriers containing iron are a tool for mitigating the spread of contaminants in groundwater. Another example involves geological deposits of redox-active iron minerals that may be used to store or produce hydrogen as a clean energy source.
Outside the Earth sciences, potential applications of iron-57-labeled synthetic minerals exist in fields as diverse as corrosion science, construction engineering, and experimental archaeology. The formation of rust on iron-bearing objects is the outcome of many interrelated chemical processes. Iron-57 tracers may help to follow and unravel those corrosion processes. They could also probe effects of different metal alloy compositions and exposure environments in tests of the longevity of steel infrastructure or of conservation methods for historical artifacts.
For now, our findings from the synthetic iron minerals we’ve “planted” in rice paddy soils are shaping understanding of the chemistry of periodically flooded soils, revealing that just like plants, the life cycles of iron minerals depend on the composition of and conditions in the soil. With continuing research in soils and with new applications focused on other natural and engineered environments, scientists can gain needed insights into how iron in its many forms affects vital issues from soil health and pollution transport to carbon storage and energy production.
Acknowledgments
This research was carried out as part of the European Research Council–funded IRMIDYN (Iron Mineral Dynamics in Soils and Sediments) project at ETH Zurich. The research was led by Ruben Kretzschmar with team members Laurel ThomasArrigo, Katherine Rothwell, Luiza Notini, Katrin Schiedung (published as Katrin Schulz), Joëlle Kubeneck, Andrew Grigg, Pierre Lefebvre, Sara Martinengo, and Giulia Fantappiè, while each was affiliated with the Institute of Biogeochemistry and Pollutant Dynamics at ETH Zurich, in Switzerland. We acknowledge important contributions to the research made by Kurt Barmettler (ETH Zurich) and Worachart Wisawapipat (Kasetsart University).
Author Information
Andrew R. C. Grigg ([email protected]), Institute of Biogeochemistry and Pollutant Dynamics, ETH Zurich, Zurich, Switzerland; Katrin Schiedung, Thünen Institute, Braunschweig, Germany; Joëlle Kubeneck, TNO–Geological Survey of the Netherlands, Utrecht; also at Radboud University, Nijmegen, Netherlands; and Ruben Kretzschmar, Institute of Biogeochemistry and Pollutant Dynamics, ETH Zurich, Zurich, Switzerland


